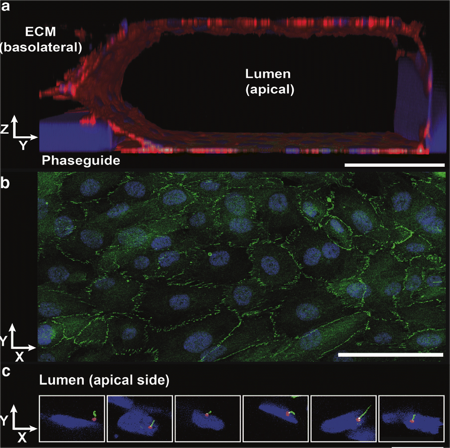
Robust validated proximal tubule-on-a-chip
Drug-induced organ toxicity accounts for 30% of all drugs that fail prior to reaching the market. Within this, nephrotoxicity accounts for 2% of failures in the preclinical stages and 19% of all failures in Phase III. There is a significant translational gap between the preclinical models of nephrotoxicity and their predictive value through the clinical stages of drug development.
Renal proximal tubules are a major target for drug-induced kidney injury (DIKI) that needs evaluation during drug development. Two-dimensional in vitro proximal tubule epithelial cell (PTEC) models are often poor predictors of DIKI, because of the lack of physiological architecture and flow. The team at MIMETAS and Radboud University Medical Centre developed ‘NephroScreen‘ containing of polarized human proximal tubule cells in the OrganoPlate, as a better predictive in vitro system for drug-induced kidney toxicity (DIKI).
In this application note, we explain more about Nephroscreen and elaborate on why it’s an ideal model to study the potential toxicity of drugs and other substances involving active uptake via these transporters.
Learn more about:
- A robust and validated 3D organ-on-a-chip proximal tubule
- The wide range of compatible readouts, showing identification of nephrotoxicants in line with (pre)clinical compound profiles
- Why it’s an ideal model to study the potential toxicity of drug substances involving active uptake via both organic anion and organic cation transporters
Want to learn more? Download the app note here.